Research group of Prof. Dr. Junker
Research team: The group of Prof. Junker is interested in several research areas combining organic chemistry and analytical chemistry methods. The main interest of the group is to understand the mode of drug action and delivery in biological systems. Organic synthesis is used to optimize mass spectrometry (MALDI-MS) methods and to modify small molecules for improved drug delivery and to use them as marker systems. Head of the group: Prof. Junker started his research career in physical organic chemistry where he examined host-guest interactions with biophysical methods. During his PhD (Dr. rer. nat.) thesis at RWTH Aachen he was involved in the synthesis of potential enzyme inhibitors and developed methods for the radical promoted C-C bond formation between glycosyl radicals and michael systems. During his postdoctoral research at MIT under the guidance of Prof. Joanne Stubbe in Cambridge, Massachusetts (USA) he worked on the better understanding of the mechanism of DNA damage by natural products. In his stage in biotech industry (Graffinity, Heidelberg) he used parallel synthesis methods to develop compound libraries for chemical microarrays. Since 2007 he is professor of organic chemistry at Aalen University. His personal interests are playing chess, tennis, music, arts and gardening.
Research Statement of Prof. Junker: My adviser at MIT JoAnne Stubbe often told me: “We are chemists,-we can do everything”. I adapted the interdisciplinary approach of JoAnne to tackle difficult problems with a broad range of methods from different areas of chemistry. I am quite happy that the excellent technical infrastructure at Aalen University enables and facilitates this approach. My coworkers get hands-on experience with high-tech analytical instruments in my lab: Doing their own measurements with nuclear magnetic resonance (DRX 500, Bruker) and mass spectrometry (MALDI-MS, Ultraflex Bruker) instruments. Beside this we are fully equipped for doing organic synthesis. Besides classical methods we use modern approaches like microwave synthesis and parallel chemistry. For purification we use low and medium pressure column chromatography with fraction collectors and UV-VIS detection. For high throughput we use preparative and analytical HPLC-MS (Shimadzu LC-MS with UV/VIS, dynamic light scattering and ESI-MS detection).
Current research projects:
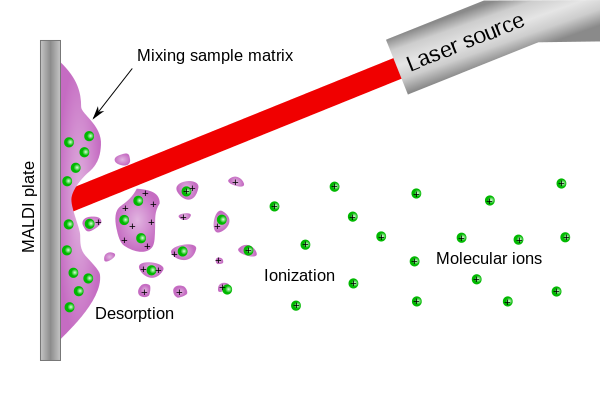
1) Development of new MALDI-MS matrices. In the Center for Applied Biomedical Mass Spectrometry (ABIMAS: http://www.zafh-abimas.de/) several research groups from Mannheim University and Aalen University work on the development of new methods for biomedical imaging using mass spectrometry. This project is founded by the European Commission and the government of Baden-Württemberg. In the subproject executed at Aalen University new methods for matrix assisted laser desorption & ionization mass spectrometry (MALDI-MS) are developed. In MALDI-MS the analyte is mixed with a matrix, which is usually a small organic compound. The matrix is used in a large excess and cocrystallizes with the analyte forming a compound cluster (see Figure 1). The next step is the energy transfer from the laser beam to the matrix compound leading to desorption and ionization of the matrix-analyte aggregate. In the gas phase this cluster disintegrates and releases the ionized analyte. This process is very gentle for the analyte, the energy is mainly transferred to the matrix. Therefore this method is exceptional suited for the analysis of fragile and large biomolecules like proteins or DNA, which stay intact. This soft ionization process has the great advantage that it can be used for the two dimensional scanning of surfaces of e.g. human tissues. But unfortunately this process relies on several complicated parameters making it difficult to predict.
Figure 1: The MALDI ionization process (taken from Wikipedia commons)
One important parameter for the optimization of this process is the choice of the right matrix. Until today only a handful of matrices are in use. These common matrices were selected by analytical chemists who had no access to organic chemistry resources. Therefore not much is known about the relationships between matrix structure and their performance as a MALDI matrix. The goal of the subproject in Aalen is to resolve this issue by a combination of organic synthesis of structurally related matrix compounds and their evaluation by screening methods. During the course of this project several hundreds of compounds were evaluated against a large number of different analyte groups like peptides, glycopeptides, proteins, natural products and lipids. This led to the discovery of several new matrices with improved properties and performance in comparison to standard matrices. The final goal of this project is to establish structure activity relationships for the different compound classes and to define general rules for the design of MALDI matrix compounds. A typical example of the research flow in the lab can be found under: Fülöp A, Porada MB, Marsching C, Meyer B, Blott H, Tambe S, Sandhoff R, Junker HD, Hopf C, 4-Phenyl-α-cyanocinnamic acid amide: Screening for a negative ion matrix for MALDI-MS imaging of multiple lipid classes. Anal Chem. 2013 85(19):9156-63.
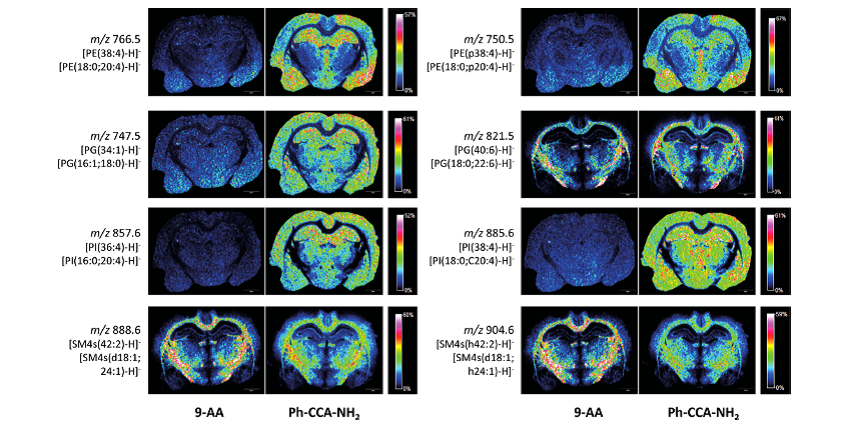
Figure 2. Superior properties of the novel MALDI-MS matrix 4-Phenyl-α-cyanocinnamic acid amide for the imaging of brain lipids in comparison to the industry standard 9-Aminoacridine. The image of brain lipids is created by using a color code for the intensity of the mass peaks of the different lipid species detected by MALDI-MS. Blue color means low intensity and red color means high intensity of the molecular ion peak of the different lipid classes (picture from Fülöp et. al.)
2) Mechanistic insight in the mode of action of the anti-cancer drug Bleomycin
Bleomycins are a group of glycopeptides from streptomyces which were first isolated in Japan from soil coming out of a coal mine. This group of antibiotics is widely used as chemotherapeutic agents to treat different kinds of cancer (e.g. head and testicular cancer, electrochemotherapy of skin metastasis of breast cancer). Bleomycins form complexes with metals like iron, copper, zinc and cobalt. Especially the iron hydroperoxide complex of bleomycin reacts in vitro in a sequence selective matter with ds-DNA leading to ds-DNA breaks. It is believed that this DNA damage process is the major cause for the activity of bleomycins as chemotherapeutic agents in the treatment of cancer.
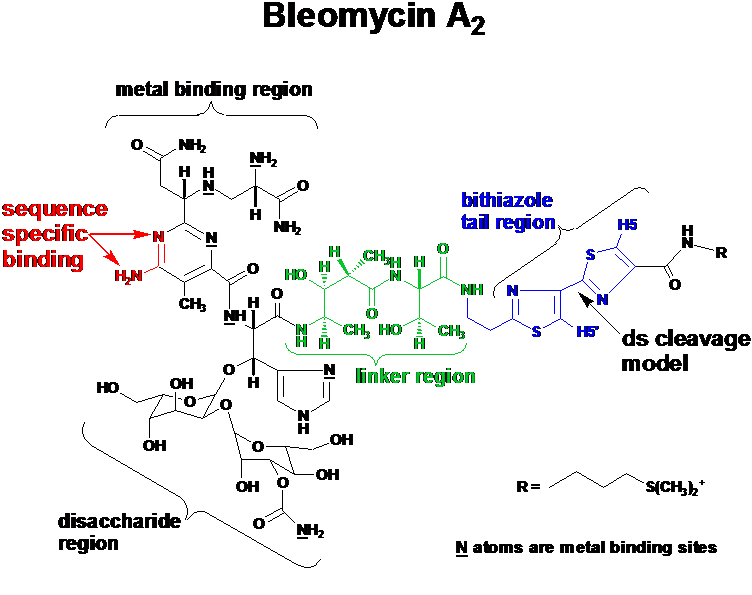
Figure 3: Structure of Bleomycin A2, a chemical endonuclease which cleaves ds-dna in a sequence selective fashion by damaging the sugar residues of the DNA using a radical based mechanism.
The molecular basis of this process is examined to a large degree. Nevertheless there is considerable doubt about the relevance of this process in vivo and about mechanistic details of the in vitro process. In vivo there are almost no free iron ions which can be picked by the metal free bleomycins. Therefore metal homeostasis in living systems must be linked in some fashion to bleomycin action. To shed some light on these aspects tools must be developed to detect bleomycin binding to metals in living systems. Typical approaches to archive this goal are the development of CE/LC-MS and MALDI-MS methods to detect these metal complexes in different surroundings. Several aspects of bleomycin research are covered in the group. One important part is the production and purification of bleomycins and their metal complexes. Fermentation/purification protocols and quality control of the fermentation/purification process by mass spectrometry and LC-MS are in development. NMR and MALDI-MS analysis of metallobleomycins and their binding against short DNA pieces will deliver important information about binding and cleavage mechanism. The final goal of this project is the development of imaging methods for metallobleomycin detection in tumor tissues. This approach will simplify the discovery of novel bleomycin derivatives with improved properties.